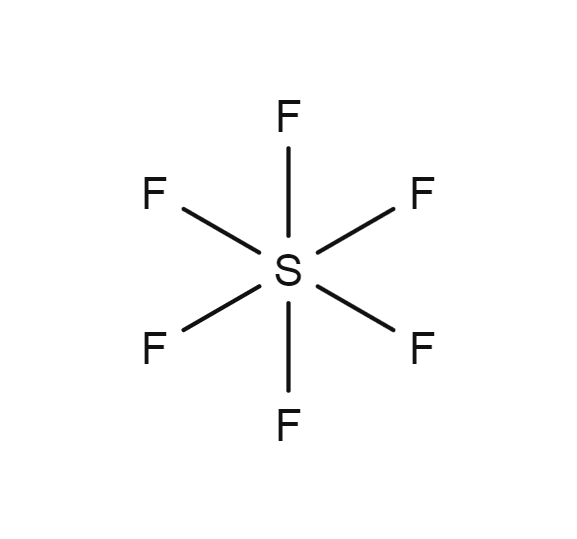
Sulfur hexafluoride
- SF6
- CAS Number 2551-62-4
- UN1080 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 146.055 g/mol
- Content in dry air /
-
Critical Point
- Temperature 45.54 °C
- Pressure 37.6 bar
- Density 735.72 kg/m³
-
Triple Point
- Temperature -49.60 °C
- Pressure 2.314 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 34.399 kJ/kg |
| Melting point | - 50.7 °C |
Pressure 1.013 bar
| Boiling point | - 68.25 °C |
| Compressibility factor Z | 9.8491E-1 |
| Cp/Cv ratio γ | 1.1074 |
| Gas density | 6.6161 kg/m3 |
| Heat capacity Cp | 6.2783E-1 kJ/(kg.K) |
| Heat capacity Cv | 5.6695E-1 kJ/(kg.K) |
| Specific volume | 1.512E-1 m3/kg |
| Thermal conductivity | 11.627 mW/(m.K) |
| Viscosity | 1.3771E-4 Po |
| Compressibility factor Z | 9.8733E-1 |
| Cp/Cv ratio γ | 1.1017 |
| Gas density | 6.2563 kg/m3 |
| Heat capacity Cp | 6.5296E-1 kJ/(kg.K) |
| Heat capacity Cv | 5.9271E-1 kJ/(kg.K) |
| Specific volume | 1.599E-1 m3/kg |
| Thermal conductivity | 12.701 mW/(m.K) |
| Viscosity | 1.4589E-4 Po |
| Compressibility factor Z | 9.8867E-1 |
| Cp/Cv ratio γ | 1.0984 |
| Gas density | 6.0383 kg/m3 |
| Heat capacity Cp | 6.6899E-1 kJ/(kg.K) |
| Heat capacity Cv | 6.0908E-1 kJ/(kg.K) |
| Specific volume | 1.656E-1 m3/kg |
| Thermal conductivity | 13.412 mW/(m.K) |
| Viscosity | 1.5123E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare
Semiconductors
In semiconductor and display manufacturing, sulfur hexafluoride provides fluorine source for high density plasma etching without generating carbon by-products. Sulfur hexafluoride can be used for etching metal silicides, nitrides and oxides versus their metal substrates. It is also used in display fabrication for the cleaning of the Chemical Vapour Deposition (CVD) reactors.

Other
Sulfur hexafluoride is an insulating material used as a dielectric in electrical transformers.
Safety & Compatibility
GHS04
Gas under pressure
Threshold of toxicity
| PEL USA OSHA (vol) | 1000 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 6000 mg/m3 or 1000 ppm |
Odor
none
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone | Satisfactory |
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant | Satisfactory |
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Sulfur hexafluoride can be prepared from the elements through exposure of sulfur to fluorine. This was also the method used by the discoverers Henri Moissan and Paul Lebeau in 1901.