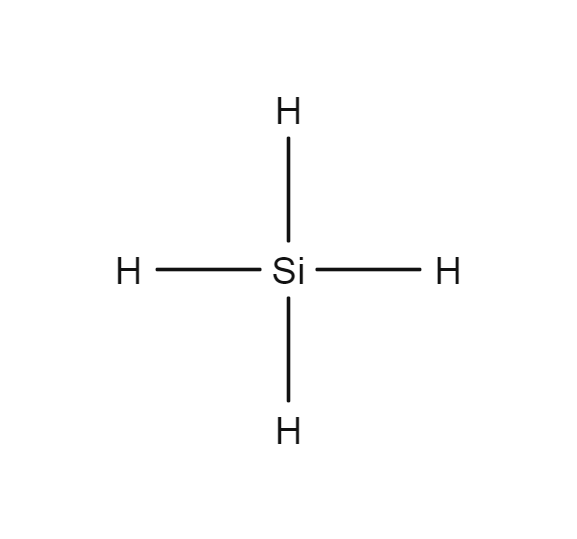
Silane
- SiH4
- CAS Number 7803-62-5
- UN2203 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 32.117 g/mol
- Content in dry air /
-
Critical Point
- Temperature -3.45 °C
- Pressure 48.4 bar
- Density 242.03 kg/m³
-
Triple Point
- Temperature -184.67 °C
- Pressure 1.9644E-4 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 20.777 kJ/kg |
| Melting point | - 184.67 °C |
Pressure 1.013 bar
| Boiling point | - 112.15 °C |
| Latent heat of vaporization (at boiling point) | 377.21 kJ/kg |
| Liquid density (at boiling point) | 582.621 kg/m3 |
| Compressibility factor Z | 9.99E-1 |
| Gas/(liquid at boiling point) equivalent | 403.76 vol/vol |
| Specific gravity | 1.12 |
| Thermal conductivity | 20.345 mW/(m.K) |
| Viscosity | 1.0704E-4 Po |
| Compressibility factor Z | 9.99E-1 |
| Gas/(liquid at boiling point) equivalent | 431.57 vol/vol |
| Specific gravity | 1.12 |
| Thermal conductivity | 21.812 mW/(m.K) |
| Viscosity | 1.1241E-4 Po |
| Compressibility factor Z | 9.99E-1 |
| Gas/(liquid at boiling point) equivalent | 431.57 vol/vol |
| Specific gravity | 1.12 |
| Thermal conductivity | 22.803 mW/(m.K) |
| Viscosity | 1.1597E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare
Semiconductors
Silane is used in semiconductor manufacturing for polysilicon and epitaxial silicon deposition, as well as the silicon source for SiO2, Si3N4, by CVD. For display fabrication, Silane is mainly used for the PECVD deposition of Si3N4 and polysilicon films. Silane is used in crystalline silicon photovoltaic cell manufacturing for the deposition of a silicon nitride antireflective layer. Silane is an intermediate for the production of high purity silicon used in photovoltaic cells and semiconductors.

Glass
Silane is an active gas for silicon containing layers coating on flat or hollow glass.
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
GHS07
Health hazards
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | Autoignitable |
| Lower flammability limit (IEC 80079-20-1) | 1.4 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | Pyrophoric |
| Lower flammability limit (NFPA 325) | 1.4 vol% |
| Upper flammability limit (NFPA 325) | 96 (est.) |
Threshold of toxicity
| VLEP 8h France (at Patm and 293.15 K) | 7 mg/m3 or 5 ppm |
Odor
Choking
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel |
Some alloys are sensitive to hydrogen embrittlement
Acceptable
|
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | No data |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | No data |
| Polypropylene | No data |
Elastomers
| Butyl (isobutene- isoprene) rubber | No data |
| Nitrile rubber | No data |
| Chloroprene | No data |
| Chlorofluorocarbons | No data |
| Silicone | No data |
| Perfluoroelastomers | No data |
| Fluoroelastomers | No data |
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant |
Contamination of the material
Not recommended
|
| Fluorocarbon based lubricant |
Contamination of the material
Not recommended
|
Materials compatibility
Learn More
More information
At normal temperature and pressure, silane is a colorless gas. It is a pyrophoric gas, which means that it undergoes spontaneous combustion in air. Above 788°F (420°C/693.15 K), silane breaks down into silicon and hydrogen. In electronics, it is often used as an active gas enabling silicon depositions and for solar panels manufacturing.