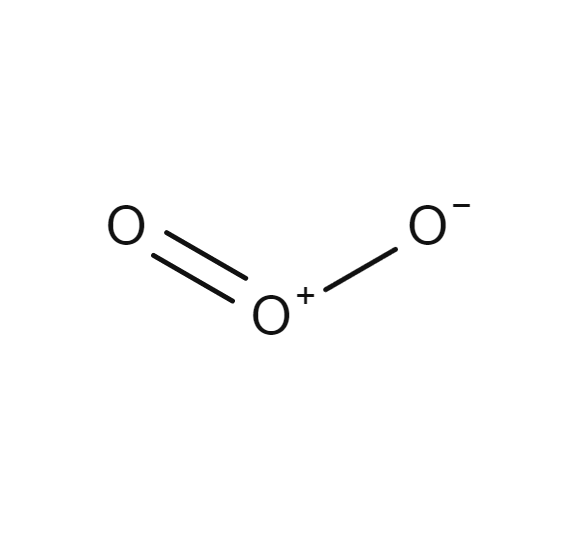
Ozone
- O3
- CAS Number 10028-15-6
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 47.998 g/mol
- Content in dry air /
-
Critical Point
- Temperature -12.15 °C
- Pressure 55.7 bar
- Density 539.31 kg/m³
-
Triple Point
- Temperature -193.00 °C
- Pressure 7.346E-6 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 41.668 kJ/kg |
| Melting point | - 193 °C |
Pressure 1.013 bar
| Boiling point | - 111.3 °C |
| Latent heat of vaporization (at boiling point) | 288.49 kJ/kg |
| Liquid density (at boiling point) | 1349.08 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 626.31 vol/vol |
| Thermal conductivity | 17.889 mW/(m.K) |
| Viscosity | 1.3923E-4 Po |
| Gas/(liquid at boiling point) equivalent | 626.31 vol/vol |
| Thermal conductivity | 19.068 mW/(m.K) |
| Viscosity | 1.4569E-4 Po |
| Gas/(liquid at boiling point) equivalent | 626.31 vol/vol |
| Thermal conductivity | 19.854 mW/(m.K) |
| Viscosity | 1.4996E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare
Electronic components
Ozone is used in (PE)ALD (Plasma Enhanced Atomic Layer Deposition) processes as an oxidant to deposit oxides. Ozone is used in some wafer cleaning steps.

Waste & Water management
Ozone is used in water management to decompose strong pollutants (through oxidation), to reduce effluent odors and color contaminants. Ozone can also be used for bleaching and is a sustainable alternative to chlore products as biocide.

Food
Ozone allows to disinfect water used for fish farming, food manufacturing and sanitation.

Other
Ozonated water is used to sanitize surfaces and disinfect products. Ozone is also used for swimming pools, spa, laundry, odor control, etc.

Pharma & Biotech
Ozone is used in chemical synthesis and for treatment of wastewater.

Pulp & Paper
Ozone allows environment-friendly paper pulp bleaching. Ozone is used to reduce the residual fluorescence coming from the optical whiteners of the waste papers. It is also used to treat specific effluents.
Safety & Compatibility
GHS03
Oxidising
GHS05
Corrosive
GHS06
Acute Toxicity
Threshold of toxicity
| PEL USA OSHA (vol) | 0.1 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 0.2 mg/m3 or 0.1 ppm |
| VLEP CT France (at Patm and 293.15 K) | 0.4 mg/m3 or 0.2 ppm |
Metals
| Aluminium | No data |
| Brass | No data |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | No data |
| Stainless steel | No data |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | No data |
| Polychlorotrifluoroethylene | No data |
| Polyvinylidene fluoride | No data |
| Polyvinyl chloride | No data |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | No data |
| Polypropylene | No data |
Elastomers
| Butyl (isobutene- isoprene) rubber | No data |
| Nitrile rubber | No data |
| Chloroprene | No data |
| Chlorofluorocarbons | No data |
| Silicone | No data |
| Perfluoroelastomers | No data |
| Fluoroelastomers | No data |
| Neoprene | No data |
| Polyurethane | No data |
| Ethylene-Propylene | No data |
Lubricants
| Hydrocarbon based lubricant | No data |
| Fluorocarbon based lubricant | No data |
Materials compatibility
Learn More
More information
Ozone was discovered in 1789 by Martin van Marum, however Christian Friedrich Schönbein is considered as the father of ozone. Its name comes from the Greek word "ozein", meaning "smell". Ozone is formed by the combination of three oxygen atoms. Ozone is an unstable gas with a strong and irritating odor (which explains its name), corrosive, very toxic and a strong oxidant. Ozone is generally produced by generating high-power electrical discharges in air or in oxygen. Naturally found in the upper layers of the atmosphere, where it is formed by a photo-chemical reaction, ozone serves as a shield which protects our planet from the sun's ultraviolet radiation.