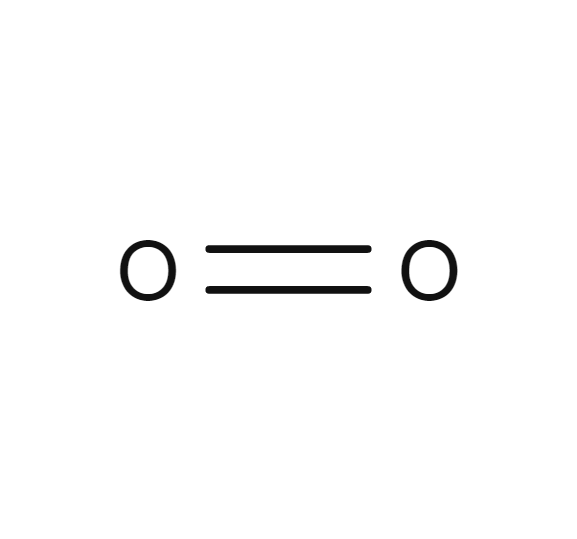
Oxygen
- O2
- CAS Number 7782-44-7
- UN1072 (gas)
- UN1073 (refrigerated liquid)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 31.999 g/mol
- Content in dry air 209460.00 ppm
-
Critical Point
- Temperature -118.57 °C
- Pressure 50.43 bar
- Density 435.95 kg/m³
-
Triple Point
- Temperature -218.79 °C
- Pressure 1.5E-3 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 13.876 kJ/kg |
| Melting point | - 218.78 °C |
Pressure 1.013 bar
| Boiling point | - 182.96 °C |
| Latent heat of vaporization (at boiling point) | 213.05 kJ/kg |
| Liquid density (at boiling point) | 1141.2 kg/m3 |
| Compressibility factor Z | 9.9903E-1 |
| Cp/Cv ratio γ | 1.3991 |
| Gas density (at boiling point) | 4.466 kg/m3 |
| Gas density | 1.4287 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 798.77 vol/vol |
| Heat capacity Cp | 9.1672E-1 kJ/(kg.K) |
| Heat capacity Cv | 6.5521E-1 kJ/(kg.K) |
| Specific gravity | 1.11 |
| Specific volume | 0.7 m3/kg |
| Thermal conductivity | 24.35 mW/(m.K) |
| Viscosity | 1.9143E-4 Po |
| Compressibility factor Z | 9.9924E-1 |
| Cp/Cv ratio γ | 1.3977 |
| Gas density | 1.354 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 843.57 vol/vol |
| Heat capacity Cp | 9.1822E-1 kJ/(kg.K) |
| Heat capacity Cv | 6.5702E-1 kJ/(kg.K) |
| Solubility in water | 2.756E-5 mol/mol |
| Specific gravity | 1.11 |
| Specific volume | 7.385E-1 m3/kg |
| Thermal conductivity | 25.55 mW/(m.K) |
| Viscosity | 1.9993E-4 Po |
| Compressibility factor Z | 9.9935E-1 |
| Cp/Cv ratio γ | 1.3967 |
| Gas density | 1.3085 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 872.14 vol/vol |
| Heat capacity Cp | 9.1962E-1 kJ/(kg.K) |
| Heat capacity Cv | 6.5846E-1 kJ/(kg.K) |
| Solubility in water | 2.293E-5 mol/mol |
| Specific gravity | 1.11 |
| Specific volume | 7.643E-1 m3/kg |
| Thermal conductivity | 26.34 mW/(m.K) |
| Viscosity | 2.055E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Aeronautics
Oxygen is used for breathing thanks to oxygen masks, in aeronautics for civil and military planes.
Automotive
Oxygen is used for laser cutting and oxycutting.

Chemicals
oxygen can be used as feedstock instead of air to improve the yield of chemical processes like oxidation reactions (e.g. prodution of ethylene oxide, propylene oxide, etc...). It is also used to debottleneck air-based processes, and brings heat through oxidation for the generation of synthesis gas (H2 + CO)
Electronic components
Oxygen is used as reactant in CVD (Chemical Vapor Deposition) and ALD (Atomic Layer Deposition) for depositing oxides. Oxygen is used to remove photoresists and amorphous carbon hard masks. Oxygen is used to grow SiO2 films on Si.

Waste & Water management
Oxygen is used instead of air to improve wastewater purification prior to discharge (aeration in biological treatment). It helps decrease power consumption, lower VOC emissions and avoid bad smells. It is also used in ozone production, a powerful oxidant applied to clean water

Food
Oxygen is used to oxygenate fish-breeding tanks. It maintains colour of fresh red meat products with Modified Atmosphere Packaging (MAP). Oxygen prevents anaerobic conditions of fresh fish and seafood products with Modified Atmosphere Packaging (MAP).

Glass
Oxycombustion: using oxygen instead of air in combustion processes limits the emission of nitrogen oxides and improve the energy efficiency

Hospital care
Oxygen is used to for respiratory diseases or intensive care

Laboratories & Research Centers
Oxygen is used in calibration gas mixtures for petrochemical industry, environmental emission monitoring, industrial hygiene monitors and trace impurity analyzers. It is also used to measure calorimetric properties of hydrocarbons and of oxidation reactions.

Metal fabrication
Oxygen is used for metals welding, cutting, and heat treatment (annealing, brazing, carburizing)

Metal
In the Iron & Steel industry, oxygen is mainly used for air enrichment in the blast furnace, decarburization in the basic oxygen furnace, and decarburization+post-combustion in the electric arc furnace. Oxygen can also be used in other plants like sinter plants and reheating furnaces. In the non-ferrous industry (Al, Cu, Pb...), Oxygen is used at different stages of the production (smelters, converters, scrap melting...).

Oil & Gas
Oxygen is used in refineries in the process of sulfur removal and for the conversion of heavy hydrocarbon fractions (in Fluid Catalytic Cracking units)

Other
Oxygen participates in bleaching operations for Pulp and Paper industry to obtain a more environmentally friendly process, essentially during the delignifying step.

Pharma & Biotech
Oxygen participates to chemical synthesis. It is used to enrich air during fermentation, as well as to treat wastewater. Oxygen is also used to flame seal glass ampuls for finished products.

Space
Liquid oxygen is used as propellant for rockets launch.
Safety & Compatibility
GHS04
Gas under pressure
GHS03
Oxidising
Odor
none
Metals
| Aluminium | Not recommended |
| Brass |
Copper alloys shall contain no more than 2.5% aluminium content
Acceptable
|
| Monel | No data |
| Copper | No data |
| Ferritic Steel |
Possible corrosion in the presence of free water
Acceptable
|
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene |
Violent reaction (oxidation/burning), dangerous product release
Not recommended
|
| Polyvinylidene fluoride |
Violent reaction (oxidation/burning), dangerous product release
Not recommended
|
| Polyvinyl chloride |
Violent reaction (oxidation/burning)
Not recommended
|
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide |
Violent reaction (oxidation/burning)
Not recommended
|
| Polypropylene |
Violent reaction (oxidation/burning)
Not recommended
|
Elastomers
| Butyl (isobutene- isoprene) rubber |
Violent reaction (oxidation/burning)
Not recommended
|
| Nitrile rubber |
Violent reaction (oxidation/burning)
Not recommended
|
| Chloroprene |
Violent reaction (oxidation/burning), dangerous product release
Not recommended
|
| Chlorofluorocarbons | No data |
| Silicone |
Violent reaction (oxidation/burning)
Not recommended
|
| Perfluoroelastomers |
Violent reaction (oxidation/burning), dangerous product release, significant swelling
Not recommended
|
| Fluoroelastomers |
Violent reaction (oxidation/burning), dangerous product release, significant swelling
Not recommended
|
| Neoprene | No data |
| Polyurethane |
Violent reaction (oxidation/burning)
Not recommended
|
| Ethylene-Propylene |
Violent reaction (oxidation/burning)
Not recommended
|
Lubricants
| Hydrocarbon based lubricant |
Violent reaction (oxidation/burning)
Not recommended
|
| Fluorocarbon based lubricant |
Violent reaction (oxidation/burning), dangerous product release
Not recommended
|
Materials compatibility
Learn More
More information
Oxygen was discovered in 1774 by Joseph Priestley. In 1777, Antoine Laurent de Lavoisier renamed "vital air" into "oxygene", from the Greek "-ὀξύς" (oxys), "acid" and "-γενής" (-genes), "producer", literally "begetter". It is the most abundant element on the earth’s surface. For example, oxygen makes up (by weight) 46 % of the Earth's crust (oxides, silicates, etc.), 89 % of the Earth's water and 62 % of the human body (in the form of molecules). In its most well-known form, it constitutes 21 % of the atmosphere. It is a tasteless, odorless and colorless gas, essential to life.