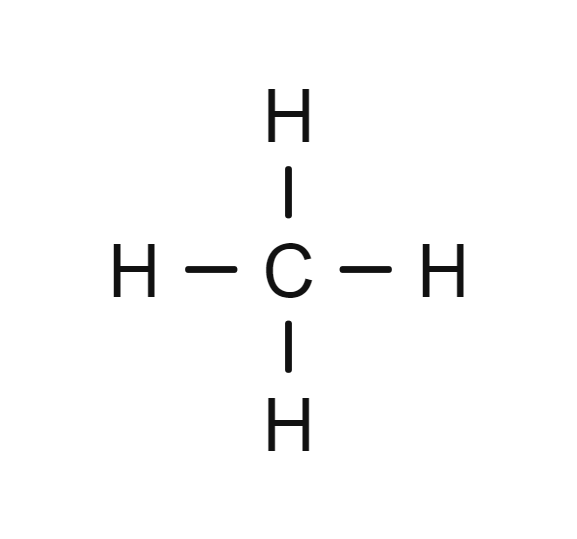
Methane
- CH4
- CAS Number 74-82-8
- UN1971 (gas)
- UN1972 (refrigerated liquid)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 16.043 g/mol
- Content in dry air 1.84 ppm
-
Critical Point
- Temperature -82.59 °C
- Pressure 45.99 bar
- Density 162.70 kg/m³
-
Triple Point
- Temperature -182.46 °C
- Pressure 1.1696E-1 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 58.682 kJ/kg |
| Melting point | - 182.46 °C |
Pressure 1.013 bar
| Boiling point | - 161.48 °C |
| Latent heat of vaporization (at boiling point) | 510.83 kJ/kg |
| Liquid density (at boiling point) | 422.36 kg/m3 |
| Compressibility factor Z | 9.9761E-1 |
| Cp/Cv ratio γ | 1.3164 |
| Gas density (at boiling point) | 1.816 kg/m3 |
| Gas density | 7.173E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 588.82 vol/vol |
| Heat capacity Cp | 2.181 kJ/(kg.K) |
| Heat capacity Cv | 1.6567 kJ/(kg.K) |
| Specific gravity | 0.56 |
| Specific volume | 1.3942 m3/kg |
| Thermal conductivity | 30.57 mW/(m.K) |
| Viscosity | 1.0245E-4 Po |
| Compressibility factor Z | 9.9802E-1 |
| Cp/Cv ratio γ | 1.3104 |
| Gas density | 6.797E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 621.4 vol/vol |
| Heat capacity Cp | 2.2094 kJ/(kg.K) |
| Heat capacity Cv | 1.6864 kJ/(kg.K) |
| Solubility in water | 3.122E-5 mol/mol |
| Specific gravity | 0.56 |
| Specific volume | 1.4713 m3/kg |
| Thermal conductivity | 32.563 mW/(m.K) |
| Viscosity | 1.0741E-4 Po |
| Compressibility factor Z | 9.9825E-1 |
| Cp/Cv ratio γ | 1.3062 |
| Gas density | 6.567E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 643.12 vol/vol |
| Heat capacity Cp | 2.2316 kJ/(kg.K) |
| Heat capacity Cv | 1.7085 kJ/(kg.K) |
| Solubility in water | 2.552E-5 mol/mol |
| Specific gravity | 0.56 |
| Specific volume | 1.5227 m3/kg |
| Thermal conductivity | 33.931 mW/(m.K) |
| Viscosity | 1.1067E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Chemicals
The main current chemical usage of methane is the production of synthesis gas (H2 and CO) via Steam Methane Reforming (SMR) or Autothermal Reforming (ATR) of natural gas. Synthesis gas can be converted into methanol, oxo-alcohols, H2 / ammonia or hydrocarbons. Methylhalogenides (e.g. methylchloride), HCN and acetylene are also produced from methane.
Electronic components
Methane provides carbon source to silicon film to adjust the light absorption bandgap in silicon thin film photovoltaics.

Hospital care
Methane is a component of gaseous mixtures used for pulmonary function diagnosis test.

Laboratories & Research Centers
Methane is used in calibration gas mixtures for Oil & Gas industry, environmental emission monitoring, industrial hygiene monitors and trace impurity analyzers. In mixture with argon, methane is used in Geiger counter and in X-ray fluorescence detector as quentching gas. In mixture with other hydrocarbons, methane is used as reference point in calorimetric measurements of hydrocarbons like Natural Gas.

Metal
Methane can be used as a reducing agent in blast furnace (partly replacing coke) and in Direct Reduced Iron (DRI) plants. The use of methane for steel making significantly reduces the CO2 footprint compared to coke-based blast furnaces.

Other
Methanol can be produced with renewable energy and serve as energy storage. It can also be used as chemical carrier to transport hydrogen.
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 595 °C |
| Lower flammability limit (IEC 80079-20-1) | 4.4 vol% |
| Upper flammability limit (IEC 80079-20-1) | 17 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 537 °C |
| Lower flammability limit (NFPA 325) | 5 vol% |
| Upper flammability limit (NFPA 325) | 15 vol% |
Odor
none
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber |
significant swelling
Not recommended
|
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone |
Significant swelling
Not recommended
|
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane |
Significant swelling
Not recommended
|
| Ethylene-Propylene |
significant swelling
Not recommended
|
Lubricants
| Hydrocarbon based lubricant | Satisfactory |
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Methane was discovered between 1776 and 1778 by Alessandro Volta. It can be found in natural gas reservoirs below ground and oceans. When in the atmosphere, it contributes to the GreenHouse effect, i.e it retains heat on earth.
