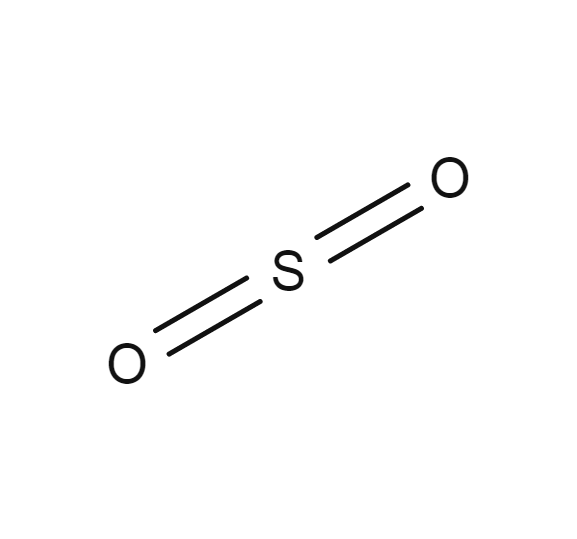
Sulfur dioxide
- SO2
- CAS Number 7446-09-5
- UN1079 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 64.064 g/mol
- Content in dry air /
-
Critical Point
- Temperature 157.60 °C
- Pressure 78.841 bar
- Density 525.11 kg/m³
-
Triple Point
- Temperature -75.45 °C
- Pressure 1.6744E-2 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 115.525 kJ/kg |
| Melting point | - 73.15 °C |
Pressure 1.013 bar
| Boiling point | - 10.02 °C |
| Latent heat of vaporization (at boiling point) | 389.06 kJ/kg |
| Liquid density (at boiling point) | 1461.1 kg/m3 |
| Compressibility factor Z | 9.7509E-1 |
| Cp/Cv ratio γ | 1.2966 |
| Gas density (at boiling point) | 3.057 kg/m3 |
| Gas density | 2.9305 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 498.58 vol/vol |
| Heat capacity Cp | 6.6921E-1 kJ/(kg.K) |
| Heat capacity Cv | 5.1614E-1 kJ/(kg.K) |
| Specific gravity | 2.26 |
| Specific volume | 3.412E-1 m3/kg |
| Thermal conductivity | 8.434 mW/(m.K) |
| Vapor pressure | 1.5637 bar |
| Viscosity | 1.1796E-4 Po |
| Compressibility factor Z | 9.8025E-1 |
| Cp/Cv ratio γ | 1.2863 |
| Gas density | 2.7633 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 528.75 vol/vol |
| Heat capacity Cp | 6.5898E-1 kJ/(kg.K) |
| Heat capacity Cv | 5.123E-1 kJ/(kg.K) |
| Solubility in water | 3.45E-2 mol/mol |
| Specific gravity | 2.26 |
| Specific volume | 3.619E-1 m3/kg |
| Thermal conductivity | 9.092 mW/(m.K) |
| Vapor pressure | 2.8104 bar |
| Viscosity | 1.2475E-4 Po |
| Compressibility factor Z | 9.8285E-1 |
| Cp/Cv ratio γ | 1.2805 |
| Gas density | 2.6636 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 548.54 vol/vol |
| Heat capacity Cp | 6.562E-1 kJ/(kg.K) |
| Heat capacity Cv | 5.1246E-1 kJ/(kg.K) |
| Solubility in water | 2.46E-2 mol/mol |
| Specific gravity | 2.26 |
| Specific volume | 3.754E-1 m3/kg |
| Thermal conductivity | 9.54 mW/(m.K) |
| Vapor pressure | 4.0015 bar |
| Viscosity | 1.2924E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Beverage
Sulfur dioxide allows to preserve wines from microbes or further oxidation during winemaking.
Electronic components
Sulfur dioxide is used as an additive to etching gas mixtures.

Food
Sulfur dioxide is used to preserve, fumigate and bleach grain.

Laboratories & Research Centers
Sulfur dioxide is used in calibration gas mixtures for petrochemical industry, environmental emission monitoring, industrial hygiene monitors and trace impurity analyzers.

Other
Sulfur dioxide is used as a flotation depressant for sulfide ores in the mining industry.

Pulp & Paper
Sulfur dioxide is used to bleach paper pulp.
Safety & Compatibility
GHS04
Gas under pressure
GHS05
Corrosive
GHS06
Acute Toxicity
Threshold of toxicity
| ILV-15min EU (at Patm and 293.15 K) | 2.7 mg/m3 or 1 ppm |
| ILV-8h EU (at Patm and 293.15 K) | 1.3 mg/m3 or 0.5 ppm |
| PEL USA OSHA (vol) | 5 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 1.3 mg/m3 or 0.5 ppm |
| VLEP CT France (at Patm and 293.15 K) | 2.7 mg/m3 or 1 ppm |
Odor
Pungent and irritating
Metals
| Aluminium | Satisfactory |
| Brass |
Risk of stress corrosion cracking in long-term wet conditions
Acceptable
|
| Monel | Satisfactory |
| Copper | No data |
| Ferritic Steel |
Sulfur dioxide hydrolyzes in presence of free water to produce sulfurous acid which is highly corrosive to steel
Acceptable
|
| Stainless steel |
Sulfur dioxide hydrolyzes in presence of free water to produce sulfurous acid which is highly corrosive to steel
Acceptable
|
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride |
Significant loss of mass
Not recommended
|
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide |
significant loss of mass
Not recommended
|
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber |
Significant loss of mass
Not recommended
|
| Chloroprene |
Significant loss of mass
Not recommended
|
| Chlorofluorocarbons | No data |
| Silicone |
Significant loss of mass
Not recommended
|
| Perfluoroelastomers |
Significant loss of mass
Not recommended
|
| Fluoroelastomers |
Significant loss of mass
Not recommended
|
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass and material contamination
Not recommended
|
| Fluorocarbon based lubricant |
material contamination
Not recommended
|
Materials compatibility
Learn More
More information
Sulfur dioxide is a noticeable component in the atmosphere, especially following volcanic eruptions.