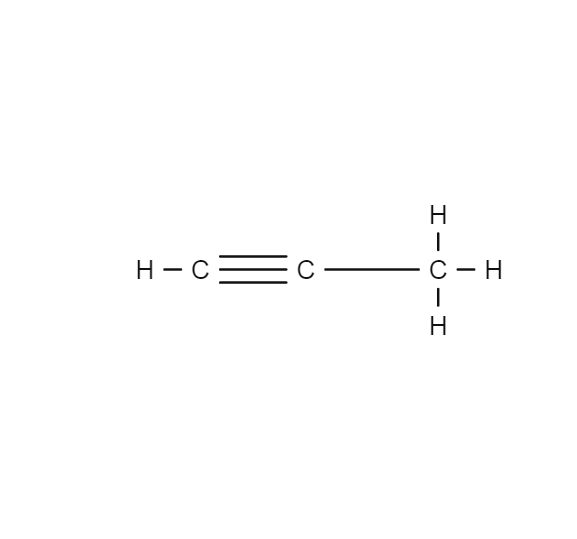
Propyne
- C3H4
- CAS Number 74-99-7
- UN1954 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 40.060 g/mol
- Content in dry air /
-
Critical Point
- Temperature 129.25 °C
- Pressure 56.3 bar
- Density 244.29 kg/m³
-
Triple Point
- Temperature -102.65 °C
- Pressure 4.171E-3 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 133.55 kJ/kg |
| Melting point | - 102.7 °C |
Pressure 1.013 bar
| Boiling point | - 25.15 °C |
| Latent heat of vaporization (at boiling point) | 553.731 kJ/kg |
| Liquid density (at boiling point) | 671.963 kg/m3 |
| Compressibility factor Z | 9.7675E-1 |
| Cp/Cv ratio γ | 1.1865 |
| Gas density | 1.8299 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 367.313 vol/vol |
| Heat capacity Cp | 1.4958 kJ/(kg.K) |
| Heat capacity Cv | 1.2607 kJ/(kg.K) |
| Specific volume | 5.465E-1 m3/kg |
| Thermal conductivity | 13.725 mW/(m.K) |
| Viscosity | 7.9079E-5 Po |
| Compressibility factor Z | 9.8092E-1 |
| Cp/Cv ratio γ | 1.1761 |
| Gas density | 1.7272 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 390.449 vol/vol |
| Heat capacity Cp | 1.5275 kJ/(kg.K) |
| Heat capacity Cv | 1.2988 kJ/(kg.K) |
| Specific volume | 5.79E-1 m3/kg |
| Thermal conductivity | 15.19 mW/(m.K) |
| Viscosity | 8.3459E-5 Po |
| Compressibility factor Z | 9.8311E-1 |
| Cp/Cv ratio γ | 1.1702 |
| Gas density | 1.6656 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 404.889 vol/vol |
| Heat capacity Cp | 1.5512 kJ/(kg.K) |
| Heat capacity Cv | 1.3256 kJ/(kg.K) |
| Specific volume | 6.004E-1 m3/kg |
| Thermal conductivity | 16.188 mW/(m.K) |
| Viscosity | 8.6348E-5 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 340 °C |
| Lower flammability limit (IEC 80079-20-1) | 1.8 vol% |
| Upper flammability limit (IEC 80079-20-1) | 16.8 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Lower flammability limit (NFPA 325) | 1.7 vol% |
Threshold of toxicity
| PEL USA OSHA (vol) | 1000 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 1650 mg/m3 or 1000 ppm |
Odor
Sweet
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide |
Significant loss of mass
Acceptable
|
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber | No data |
| Nitrile rubber | No data |
| Chloroprene | No data |
| Chlorofluorocarbons | No data |
| Silicone | No data |
| Perfluoroelastomers | No data |
| Fluoroelastomers | No data |
| Neoprene | No data |
| Polyurethane | No data |
| Ethylene-Propylene | No data |
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass and impurities in the gas
Not recommended
|
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Propyne, or methylacetylene, is an alkyne with the chemical formula CH3C≡CH. It is produced as a side product, often an undesirable one, by cracking propane to produce propene, an important feedstock in the chemical. European space companies have researched using light hydrocarbons with liquid oxygen as a relatively high performing liquid rocket propellant combination that would also be less toxic than the commonly used MMH/NTO (monomethylhydrazine/nitrogen tetroxide). Their research showed that propyne would be highly advantageous as a rocket fuel for craft intended for low Earth orbital operations. It is colorless and highly flammable.