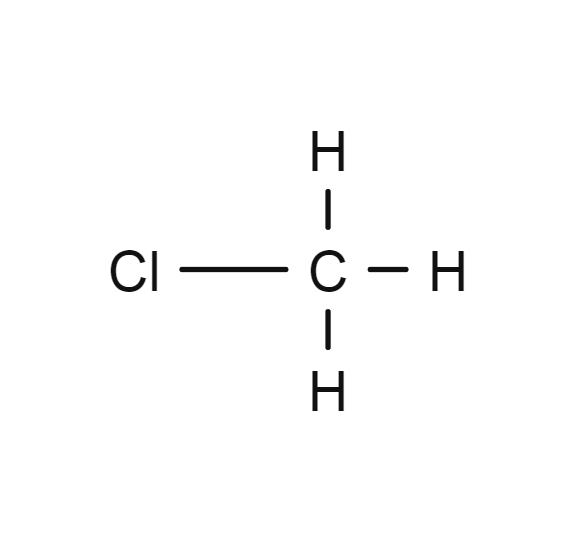
Methyl chloride
- CH3Cl
- CAS Number 74-87-3
- UN1063 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 50.488 g/mol
- Content in dry air /
-
Critical Point
- Temperature 143.10 °C
- Pressure 66.8 bar
- Density 358.07 kg/m³
-
Triple Point
- Temperature -97.72 °C
- Pressure 8.821E-3 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 127.377 kJ/kg |
| Melting point | - 97.72 °C |
Pressure 1.013 bar
| Boiling point | - 24.2 °C |
| Latent heat of vaporization (at boiling point) | 431.444 kJ/kg |
| Liquid density (at boiling point) | 1004.3 kg/m3 |
| Gas density | 2.3083 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 435.42 vol/vol |
| Specific gravity | 1.8 |
| Thermal conductivity | 9.412 mW/(m.K) |
| Vapor pressure | 2.599 bar |
| Viscosity | 1.019E-4 Po |
| Gas density (at boiling point) | 2.5595 kg/m3 |
| Gas density | 2.1782 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 435.42 vol/vol |
| Specific gravity | 1.8 |
| Thermal conductivity | 10.32 mW/(m.K) |
| Vapor pressure | 4.272 bar |
| Viscosity | 1.0697E-4 Po |
| Gas density | 2.1001 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 435.42 vol/vol |
| Specific gravity | 1.8 |
| Thermal conductivity | 10.941 mW/(m.K) |
| Vapor pressure | 5.768 bar |
| Viscosity | 1.1033E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Chemicals
Methyl chloride is used mainly in the production of silicones. Methyl chloride is also used in the production of agricultural chemicals, methyl cellulose, quaternary amines and butyl rubber.
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
GHS08
Serious health hazard
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 625 °C |
| Lower flammability limit (IEC 80079-20-1) | 7.6 vol% |
| Upper flammability limit (IEC 80079-20-1) | 19 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 632 °C |
| Flash point (NPFA 325) | - 50 °C |
| Lower flammability limit (NFPA 325) | 8.1 vol% |
| Upper flammability limit (NFPA 325) | 17.4 vol% |
Threshold of toxicity
| PEL USA OSHA (vol) | 100 ppm |
Odor
Faintly sweet
Metals
| Aluminium | Not recommended |
| Brass | Satisfactory |
| Monel | Satisfactory |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride |
Significant swelling
Not recommended
|
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene |
Significant swelling
Not recommended
|
Elastomers
| Butyl (isobutene- isoprene) rubber |
Significant swelling
Not recommended
|
| Nitrile rubber |
Significant swelling
Not recommended
|
| Chloroprene |
Significant swelling
Not recommended
|
| Chlorofluorocarbons | No data |
| Silicone |
Significant swelling
Not recommended
|
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane |
Significant swelling
Not recommended
|
| Ethylene-Propylene |
Significant swelling
Not recommended
|
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass
Not recommended
|
| Fluorocarbon based lubricant |
Significant loss of mass
Not recommended
|
Materials compatibility
Learn More
More information
Due to their ozone-depleting effect, the production of refrigerants is continuously decreasing, based on Montreal protocol requirements. Their use is controlled and they are progressively being replaced.