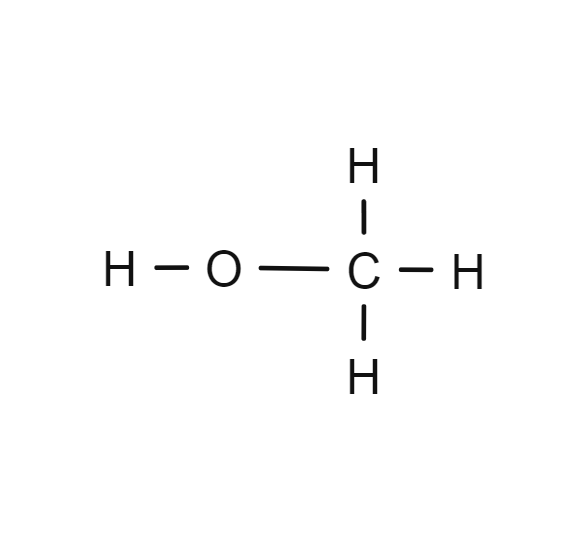
Methanol
- CH3OH
- CAS Number 67-56-1
- UN1230 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 32.042 g/mol
- Content in dry air /
-
Critical Point
- Temperature 239.35 °C
- Pressure 80.84 bar
- Density 273.86 kg/m³
-
Triple Point
- Temperature -97.54 °C
- Pressure 1.86E-6 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 100.34 kJ/kg |
| Melting point | - 97.68 °C |
Pressure 1.013 bar
| Boiling point | 64.48 °C |
| Latent heat of vaporization (at boiling point) | 1101.1 kJ/kg |
| Liquid density (at boiling point) | 748.36 kg/m3 |
Applications
Examples of uses of this molecule in Industry and Healthcare
Automotive
Methanol is used for the heat treatment of metallic pieces in vehicles. Methanol can also be used as fuel, for instance in blend with gasoline.

Chemicals
Methanol is widely used in the chemical industry to produce other chemicals. It is used for the synthesis of resins, acetic acid, olefins such as ethylene and propylene, or formaldehyde.

Laboratories & Research Centers
Methanol is used as solvant in liquid chromatography and UV spectroscopy.

Metal fabrication
Methanol is used for the heat treatment of metallic products.
Safety & Compatibility
GHS02
Flammable
GHS06
Acute Toxicity
GHS08
Serious health hazard
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 440 °C |
| Flash point (Chemsafe) | 9 °C |
| Lower flammability limit (IEC 80079-20-1) | 6 vol% |
| Upper flammability limit (IEC 80079-20-1) | 50 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 464 °C |
| Flash point (NPFA 325) | 11 °C |
| Lower flammability limit (NFPA 325) | 6 vol% |
| Upper flammability limit (NFPA 325) | 36 vol% |
Threshold of toxicity
| ILV-8h EU (at Patm and 293.15 K) | 260 mg/m3 or 200 ppm |
| PEL USA OSHA (vol) | 200 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 260 mg/m3 or 200 ppm |
| VLEP CT France (at Patm and 293.15 K) | 1300 mg/m3 or 1000 ppm |
Metals
| Aluminium | No data |
| Brass | No data |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | No data |
| Stainless steel | No data |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | No data |
| Polychlorotrifluoroethylene | No data |
| Polyvinylidene fluoride | No data |
| Polyvinyl chloride | No data |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | No data |
| Polypropylene | No data |
Elastomers
| Butyl (isobutene- isoprene) rubber | No data |
| Nitrile rubber | No data |
| Chloroprene | No data |
| Chlorofluorocarbons | No data |
| Silicone | No data |
| Perfluoroelastomers | No data |
| Fluoroelastomers | No data |
| Neoprene | No data |
| Polyurethane | No data |
| Ethylene-Propylene | No data |
Lubricants
| Hydrocarbon based lubricant | No data |
| Fluorocarbon based lubricant | No data |
Materials compatibility
Learn More
More information
Methanol was isolated for the first time in 1661 by Robert Boyle. He gives him the name of "spirit of the woods." In 1834, Jean Baptiste Dumas and Eugene Melchior called it "methylene" in connection with Greek words: "methy" (wine) and "hyle" (wood).