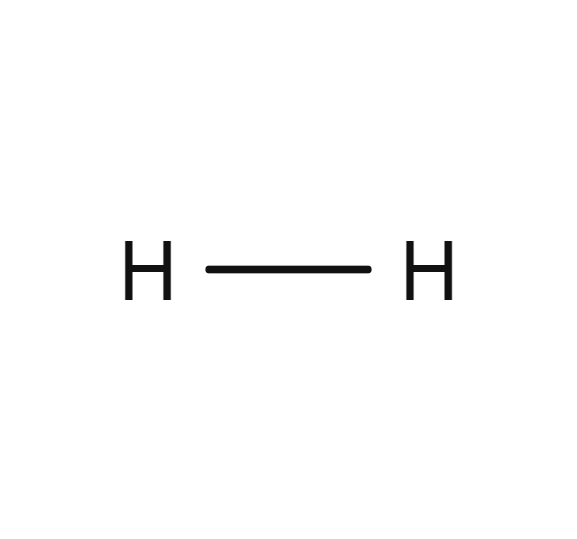
Hydrogen
- H2
- CAS Number 1333-74-0
- UN1049 (gas)
- UN1966 (refrigerated liquid)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 2.016 g/mol
- Content in dry air /
-
Critical Point
- Temperature -239.96 °C
- Pressure 13.13 bar
- Density 31.43 kg/m³
-
Triple Point
- Temperature -259.19 °C
- Pressure 7.7E-2 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 58.089 kJ/kg |
| Melting point | - 259.2 °C |
Pressure 1.013 bar
| Boiling point | - 252.78 °C |
| Latent heat of vaporization (at boiling point) | 448.69 kJ/kg |
| Liquid density (at boiling point) | 70.516 kg/m3 |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.4098 |
| Gas density (at boiling point) | 1.438 kg/m3 |
| Gas density | 8.99E-2 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 785.24 vol/vol |
| Heat capacity Cp | 14.1976 kJ/(kg.K) |
| Heat capacity Cv | 10.0704 kJ/(kg.K) |
| Specific gravity | 0.07 |
| Specific volume | 11.128 m3/kg |
| Thermal conductivity | 172.58 mW/(m.K) |
| Viscosity | 8.3969E-5 Po |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.4069 |
| Gas density | 8.52E-2 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 831.17 vol/vol |
| Heat capacity Cp | 14.2676 kJ/(kg.K) |
| Heat capacity Cv | 10.1409 kJ/(kg.K) |
| Solubility in water | 1.51E-5 mol/mol |
| Specific gravity | 0.07 |
| Specific volume | 11.739 m3/kg |
| Thermal conductivity | 180.05 mW/(m.K) |
| Viscosity | 8.7098E-5 Po |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.4054 |
| Gas density | 8.23E-2 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 857.09 vol/vol |
| Heat capacity Cp | 14.3063 kJ/(kg.K) |
| Heat capacity Cv | 10.1796 kJ/(kg.K) |
| Solubility in water | 1.411E-5 mol/mol |
| Specific gravity | 0.07 |
| Specific volume | 11.983 m3/kg |
| Thermal conductivity | 184.88 mW/(m.K) |
| Viscosity | 8.9154E-5 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Aeronautics
Aviation companies are currently testing and examining the potential for using hydrogen to replace the Auxiliary Power Units (APUs) and use fuel cells to generate electrical power for non-essential aircraft applications.
Automotive
Hydrogen is an essential vector to decarbonize the mobility sector, by using electric vehicles fed by a fuel cell transforming hydrogen into electricity . Hydrogen is stored in the stations and on the vehicles in advanced hydrogen tanks, in liquid form or as gas compressed at high pressure. Today hydrogen is very interesting for captured fleets (forklifts, buses, airport traffic) and passenger cars, and development for trains, trucks, ships and even airmobility are ongoing. Hydrogen is also used for metal heat treatment in vehicles, and used in mixtures for arc and plasma welding of stainless steels.

Chemicals
Hydrogen is an essential compound to generate valuable products such as dimethyl ether (DME) and propylene that are important sources of industrial chemicals and plastic products. Hydrogen is also used for the synthesis of ammonia and other amines (e.g. aniline) or alkanes (by hydrogenation of alkenes). In combination with carbon monoxide in so-called synthesis gas, hydrogen is used for methanol and synthetic fuel production.
Electronic components
Hydrogen is used in Silicon epitaxy processes. Hydrogen is also used in EUV (Extrem Ultraviolet) tools to minimize Sn deposition, as well as a co-reactant in (PE)ALD processes (Plasma Enhanced Atomic Layer Deposition).

Food
Hydrogen is used for the hydrogenation of amines and fatty acids for food solid fats production.

Glass
Hydrogen mixed with nitrogen creates a reductive atmosphere over the tin bath in glass production process preventing oxygen to react with the tin. Hydrogen is used in heat treatment (oxy-hydrogen flame) of hollow glass and optic fibers pre-forms.

Laboratories & Research Centers
Hydrogen is used for analysis in research laboratories and quality control for industry and healthcare. Hydrogen is a carrier gas in gas chromatography and in various analytical instrument applications (flame ionization detector).

Metal
Hydrogen, mixed with protective inert gases (H2 diluted in nitrogen) eliminates all oxygen traces, harmful for these medium and high temperature processes. Hydrogen, mixed with nitrogen, is used for metal heat treatment (heating operations applied to metals - sheets, tubes, wires or parts - to improve their mechanical properties). Hydrogen is also used in mixtures for arc or plasma welding of stainless steels.

Oil & Gas
Hydrogen is used in refinery to remove sulfur from petroleum products (hydrodesulfurization process) to reduce sulfur dioxide emissions during fuel combustion and prevent acid rain. Hydrogen is used in many other units in refineries : to crack long-chain hydrocarbons into lighter hydrocarbons (hydrocracking process), to convert normal paraffins into iso-paraffins and improve the product properties (hydroisomerisation) and to remove aromatic compounds from a mixture, especially as part of the oil refining process (dearomatisation).

Other
Hydrogen fuel cells can be used as clean and autonomous power supply for off-grid sites, such as telecommunication stations and to power residential homes.

Photonics
Hydrogen is used in the fabrication of fibre optic as a high purity gas.

Space
Liquid hydrogen is used as rocket or launcher propellant. In combination with an oxidizer such as liquid oxygen, liquid hydrogen yields the highest specific impulse, or efficiency in relation to the amount of propellant consumed of any known rocket propellant.
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 560 °C |
| Lower flammability limit (IEC 80079-20-1) | 4 vol% |
| Upper flammability limit (IEC 80079-20-1) | 77 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 500 °C |
| Lower flammability limit (NFPA 325) | 4 vol% |
| Upper flammability limit (NFPA 325) | 75 vol% |
Odor
none
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel |
Risk of hydrogen embrittlement
Acceptable
|
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene |
Strong rate of permeation
Acceptable
|
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene |
Strong rate of permeation
Acceptable
|
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone |
Strong rate of permeation
Acceptable
|
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant | Satisfactory |
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Hydrogen was discovered by Henry Cavendish in 1766. It owes its name to Antoine Laurent de Lavoisier, who combined the Greek "hydor", "water", and "genen", "to engender". Hydrogen is colorless, very light, and reacts easily with other chemical substances making it a very useful reactant for the chemical and oil industries. Hydrogen is found in the atmosphere at trace levels. In 1970, hydrogen fluxes have been detected in the oceans, resulting from serpentinization of rocks but the exploitation seems unlikely due to extreme geological conditions. More recently, natural hydrogen emissions from the ground have also been detected and are under investigation to understand the origin of this hydrogen. Today natural gas or hydrocarbons as well as water are used as raw materials to produce hydrogen. Moreover, hydrogen has a very high energy content (120 MJ/kg) while containing no carbon atom. This makes it a promising energy vector for the future. Associated to air or oxygen in a fuel cell, hydrogen is converted in electricity and heat, releasing only water. Driving an electric car over a long distance with no emission is a dream of many !