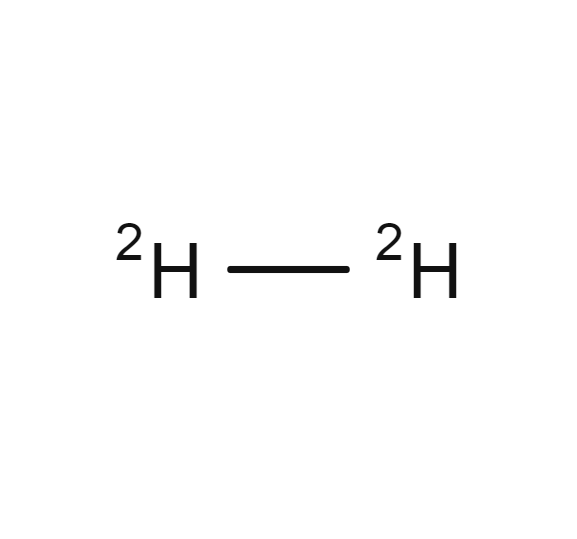
Deuterium
- D2
- CAS Number 7782-39-0
- UN1957 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 4.032 g/mol
- Content in dry air /
-
Critical Point
- Temperature -234.80 °C
- Pressure 16.617 bar
- Density 66.90 kg/m³
-
Triple Point
- Temperature -254.44 °C
- Pressure 1.707E-1 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 49.261 kJ/kg |
| Melting point | - 254.42 °C |
Pressure 1.013 bar
| Boiling point | - 249.84 °C |
| Latent heat of vaporization (at boiling point) | 322.215 kJ/kg |
| Liquid density (at boiling point) | 163.83 kg/m3 |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.3983 |
| Gas density (at boiling point) | 2.105 kg/m3 |
| Gas density | 1.796E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 894.03 vol/vol |
| Heat capacity Cp | 7.2499 kJ/(kg.K) |
| Heat capacity Cv | 5.1847 kJ/(kg.K) |
| Specific volume | 5.5691 m3/kg |
| Thermal conductivity | 130.07 mW/(m.K) |
| Viscosity | 1.1823E-4 Po |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.3982 |
| Gas density | 1.702E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 943.09 vol/vol |
| Heat capacity Cp | 7.2509 kJ/(kg.K) |
| Heat capacity Cv | 5.1859 kJ/(kg.K) |
| Solubility in water | 1.595E-5 mol/mol |
| Specific volume | 5.8748 m3/kg |
| Thermal conductivity | 135.04 mW/(m.K) |
| Viscosity | 1.2267E-4 Po |
| Compressibility factor Z | 1.0006 |
| Cp/Cv ratio γ | 1.3981 |
| Gas density | 1.645E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 975.82 vol/vol |
| Heat capacity Cp | 7.2514 kJ/(kg.K) |
| Heat capacity Cv | 5.1865 kJ/(kg.K) |
| Solubility in water | 1.46E-5 mol/mol |
| Specific volume | 6.0786 m3/kg |
| Thermal conductivity | 138.2 mW/(m.K) |
| Viscosity | 1.256E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare
Electronic components
Deuterium is used as an alternative to hydrogen in the annealing process to improve carrier mobility.

Other
Deuterium is used in nuclear fusion reactions.

Pharma & Biotech
Deuterium is used to prepare deuterated compounds in chemistry and biochemistry. These tracer molecules allow to study reaction rates and mechanisms of the reaction.
Safety & Compatibility
GHS02
Flammable
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 560 °C |
| Lower flammability limit (IEC 80079-20-1) | 6.7 vol% |
| Upper flammability limit (IEC 80079-20-1) | 79.6 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Lower flammability limit (NFPA 325) | 5 vol% |
| Upper flammability limit (NFPA 325) | 75 vol% |
Odor
none
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel |
Risk of hydrogen embrittlement
Acceptable
|
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene |
Risk of permeation
Acceptable
|
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene |
Risk of permeation
Acceptable
|
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone |
Risk of permeation
Acceptable
|
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant | Satisfactory |
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Deuterium was discovered spectroscopically in 1931 by Harold Clayton Urey, which earned him the Nobel Prize in chemistry in 1934. Deuterium, or “heavy hydrogen”, is the isotope of hydrogen of atomic weight 2.