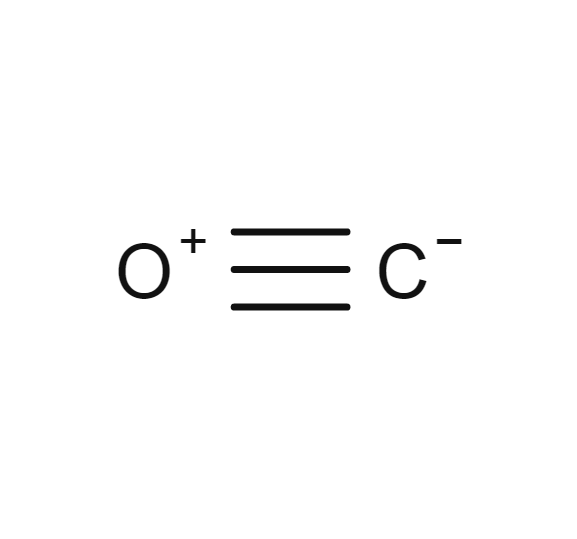
Carbon monoxide
- CO
- CAS Number 630-08-0
- UN1016 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 28.010 g/mol
- Content in dry air /
-
Critical Point
- Temperature -140.23 °C
- Pressure 34.99 bar
- Density 296.72 kg/m³
-
Triple Point
- Temperature -204.99 °C
- Pressure 1.53E-1 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 30.024 kJ/kg |
| Melting point | - 205.07 °C |
Pressure 1.013 bar
| Boiling point | - 191.5 °C |
| Latent heat of vaporization (at boiling point) | 214.68 kJ/kg |
| Liquid density (at boiling point) | 793.2 kg/m3 |
| Compressibility factor Z | 9.9934E-1 |
| Cp/Cv ratio γ | 1.4021 |
| Gas density (at boiling point) | 4.36 kg/m3 |
| Gas density | 1.2502 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 634.46 vol/vol |
| Heat capacity Cp | 1.042 kJ/(kg.K) |
| Heat capacity Cv | 7.4316E-1 kJ/(kg.K) |
| Specific gravity | 0.97 |
| Specific volume | 7.999E-1 m3/kg |
| Thermal conductivity | 24.74 mW/(m.K) |
| Viscosity | 1.6515E-4 Po |
| Compressibility factor Z | 9.9953E-1 |
| Cp/Cv ratio γ | 1.4016 |
| Gas density | 1.1849 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 669.42 vol/vol |
| Heat capacity Cp | 1.042 kJ/(kg.K) |
| Heat capacity Cv | 7.4345E-1 kJ/(kg.K) |
| Solubility in water | 2.095E-5 mol/mol |
| Specific gravity | 0.97 |
| Specific volume | 8.44E-1 m3/kg |
| Thermal conductivity | 25.79 mW/(m.K) |
| Viscosity | 1.7201E-4 Po |
| Compressibility factor Z | 9.9964E-1 |
| Cp/Cv ratio γ | 1.4013 |
| Gas density | 1.145 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 692.75 vol/vol |
| Heat capacity Cp | 1.0421 kJ/(kg.K) |
| Heat capacity Cv | 7.437E-1 kJ/(kg.K) |
| Solubility in water | 1.774E-5 mol/mol |
| Specific gravity | 0.97 |
| Specific volume | 8.734E-1 m3/kg |
| Thermal conductivity | 26.478 mW/(m.K) |
| Viscosity | 1.7649E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Chemicals
Carbon monoxide is an intermediate component used for the synthesis of different valuable products like plastics. As component of the so-called synthetic gas (H2 + CO) it is used to produce methanol and synthetic fuels.
Electronic components
Carbon Monoxide is used is some plasma etching processes.

Food
Carbon monoxide maintains colour of fresh red meat products with Modified Atmosphere Packaging (MAP).

Hospital care
Carbon monoxyde is a component of gaseous mixtures used for pulmonary function diagnosis tests.

Laboratories & Research Centers
Carbon monoxide is used in calibration gas mixtures for petrochemical industry, environmental emission monitoring, industrial hygiene monitors and trace impurity analyzers.

Pharma & Biotech
Carbon monoxide is used as a reactant in some chemical synthesis processes (e.g. carbonylation reactions).
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
GHS06
Acute Toxicity
GHS08
Serious health hazard
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 605 °C |
| Lower flammability limit (IEC 80079-20-1) | 10.9 vol% |
| Upper flammability limit (IEC 80079-20-1) | 76 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 609 °C |
| Lower flammability limit (NFPA 325) | 12.5 vol% |
| Upper flammability limit (NFPA 325) | 74 vol% |
Threshold of toxicity
| ILV-15min EU (at Patm and 293.15 K) | 117 mg/m3 or 100 ppm |
| ILV-8h EU (at Patm and 293.15 K) | 23 mg/m3 or 20 ppm |
| PEL USA OSHA (vol) | 50 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 23 mg/m3 or 20 ppm |
| VLEP CT France (at Patm and 293.15 K) | 117 mg/m3 or 100 ppm |
Odor
none
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel |
Risk of stress corrosion cracking
Acceptable
|
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber |
Notable acceleration of the process of ageing
Acceptable
|
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone | Satisfactory |
| Perfluoroelastomers |
Significant swelling
Acceptable
|
| Fluoroelastomers |
Significant swelling
Acceptable
|
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant | Satisfactory |
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
Carbon monoxide is formed from the combination of a carbon atom with an oxygen atom. Not only flammable, it is also very hazardous since it is very toxic and odorless. It is produced, among other ways, from incomplete combustion due to lack of oxygen. It can therefore cause domestic accidents due to poorly maintained heating systems.