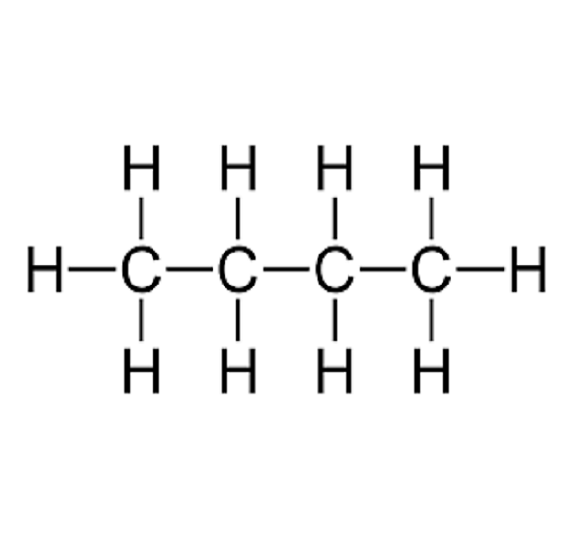
n-Butane
- C4H10
- CAS Number 106-97-8
- UN1011 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 58.122 g/mol
- Content in dry air /
-
Critical Point
- Temperature 151.97 °C
- Pressure 37.96 bar
- Density 227.93 kg/m³
-
Triple Point
- Temperature -138.26 °C
- Pressure 6.736E-6 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 80.193 kJ/kg |
| Melting point | - 138.29 °C |
Pressure 1.013 bar
| Boiling point | - 0.49 °C |
| Latent heat of vaporization (at boiling point) | 385.71 kJ/kg |
| Liquid density (at boiling point) | 601.26 kg/m3 |
| Compressibility factor Z | 9.5911E-1 |
| Cp/Cv ratio γ | 1.1188 |
| Gas density (at boiling point) | 2.709 kg/m3 |
| Gas density | 2.703 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 222.44 vol/vol |
| Heat capacity Cp | 1.6426 kJ/(kg.K) |
| Heat capacity Cv | 1.4682 kJ/(kg.K) |
| Specific gravity | 2.08 |
| Specific volume | 0.37 m3/kg |
| Thermal conductivity | 14.189 mW/(m.K) |
| Vapor pressure | 1.0339 bar |
| Viscosity | 6.769E-5 Po |
| Compressibility factor Z | 9.6616E-1 |
| Cp/Cv ratio γ | 1.1102 |
| Gas density | 2.5436 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 236.38 vol/vol |
| Heat capacity Cp | 1.6944 kJ/(kg.K) |
| Heat capacity Cv | 1.5263 kJ/(kg.K) |
| Solubility in water | 3.274E-5 mol/mol |
| Specific gravity | 2.08 |
| Specific volume | 3.931E-1 m3/kg |
| Thermal conductivity | 15.59 mW/(m.K) |
| Vapor pressure | 1.7644 bar |
| Viscosity | 7.1524E-5 Po |
| Compressibility factor Z | 9.6996E-1 |
| Cp/Cv ratio γ | 1.1052 |
| Gas density | 2.4487 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 245.54 vol/vol |
| Heat capacity Cp | 1.7317 kJ/(kg.K) |
| Heat capacity Cv | 1.5668 kJ/(kg.K) |
| Solubility in water | 2.244E-5 mol/mol |
| Specific gravity | 2.08 |
| Specific volume | 4.084E-1 m3/kg |
| Thermal conductivity | 16.564 mW/(m.K) |
| Vapor pressure | 2.4368 bar |
| Viscosity | 7.4054E-5 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Laboratories & Research Centers
N-Butane is used in calibration gas mixtures for petrochemical industry, environmental emission monitoring and trace impurity analyzers.

Other
N-Butane is used as a propellant for aerosol production.
Safety & Compatibility
GHS02
Flammable
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 392 °C |
| Lower flammability limit (IEC 80079-20-1) | 1.4 vol% |
| Upper flammability limit (IEC 80079-20-1) | 9.4 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 287 °C |
| Lower flammability limit (NFPA 325) | 1.9 vol% |
| Upper flammability limit (NFPA 325) | 8.5 vol% |
Threshold of toxicity
| VLEP 8h France (at Patm and 293.15 K) | 1900 mg/m3 or 800 ppm |
Odor
Faintly disagreeable
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber |
Significant swelling
Not recommended
|
| Nitrile rubber | Satisfactory |
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone |
Significant swelling and modification of the properties of the material
Not recommended
|
| Perfluoroelastomers | Satisfactory |
| Fluoroelastomers | Satisfactory |
| Neoprene | No data |
| Polyurethane | Satisfactory |
| Ethylene-Propylene |
Significant swelling and modification of the properties of the material
Not recommended
|
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass
Not recommended
|
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Learn More
More information
n-Butane is one of the structural isomers of Butane, an organic compound with the formula C4H10 that is an alkane with four carbon atoms. The name butane comes from the roots "but-" from "butyric acid". Butane is mainly used as a fuel for domestic use (gas stove, water heater) and for extras (heating). n-Butane is prepared by pressurized distillation of liquefied petroleum gas or by purification of natural gas. It is solvable in aclohol and ether but not so much in water. Mostly inert, it needs a catalyst to participate in chimical reactions, except for combustion with dioxygen. Butanes are highly flammable, colorless, easily liquefied gases.