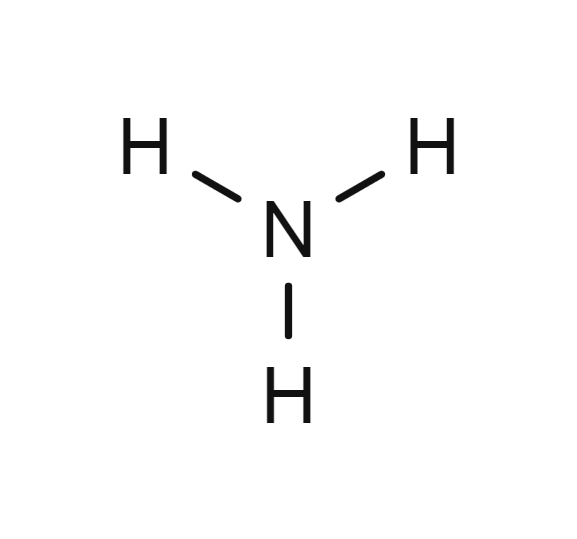
Ammonia
- NH3
- CAS Number 7664-41-7
- UN1005 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 17.030 g/mol
- Content in dry air /
-
Critical Point
- Temperature 132.50 °C
- Pressure 112.8 bar
- Density 235.00 kg/m³
-
Triple Point
- Temperature -77.66 °C
- Pressure 6.1111E-2 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 332.17 kJ/kg |
| Melting point | - 77.74 °C |
Pressure 1.013 bar
| Boiling point | - 33.33 °C |
| Latent heat of vaporization (at boiling point) | 1369.5 kJ/kg |
| Liquid density (at boiling point) | 681.97 kg/m3 |
| Compressibility factor Z | 9.8487E-1 |
| Cp/Cv ratio γ | 1.3272 |
| Gas density (at boiling point) | 8.89E-1 kg/m3 |
| Gas density | 7.713E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 884.19 vol/vol |
| Heat capacity Cp | 2.1795 kJ/(kg.K) |
| Heat capacity Cv | 1.6421 kJ/(kg.K) |
| Specific gravity | 0.6 |
| Specific volume | 1.2965 m3/kg |
| Thermal conductivity | 22.916 mW/(m.K) |
| Vapor pressure | 4.2861 bar |
| Viscosity | 9.1931E-5 Po |
| Compressibility factor Z | 9.8789E-1 |
| Cp/Cv ratio γ | 1.3203 |
| Gas density | 7.289E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 935.62 vol/vol |
| Heat capacity Cp | 2.1662 kJ/(kg.K) |
| Heat capacity Cv | 1.6407 kJ/(kg.K) |
| Specific gravity | 0.6 |
| Specific volume | 1.3719 m3/kg |
| Thermal conductivity | 24.073 mW/(m.K) |
| Vapor pressure | 7.2624 bar |
| Viscosity | 9.7289E-5 Po |
| Compressibility factor Z | 9.8946E-1 |
| Cp/Cv ratio γ | 1.316 |
| Gas density | 7.033E-1 kg/m3 |
| Gas/(liquid at boiling point) equivalent | 969.62 vol/vol |
| Heat capacity Cp | 2.1645 kJ/(kg.K) |
| Heat capacity Cv | 1.6448 kJ/(kg.K) |
| Specific gravity | 0.6 |
| Specific volume | 1.4218 m3/kg |
| Thermal conductivity | 24.934 mW/(m.K) |
| Vapor pressure | 9.9963 bar |
| Viscosity | 1.0093E-4 Po |
Applications
Examples of uses of this molecule in Industry and Healthcare

Aeronautics
Ammonia is used for heat treatment: at high temperature it splits into hydrogen and nitrogen creating the necessary reducing atmosphere.
Automotive
Ammonia is used for heat treatment: at high temperature it splits into hydrogen and nitrogen creating the necessary reducing atmosphere.
Semiconductors
Ammonia takes part of deposit silicon nitride by Chemical Vapor Deposition (CVD) in semiconductor and advanced materials manufacturing.

Waste & Water management
Ammonia can be used in emissions management to reduce the amount of nitrogen oxides (process called SCR: Selective Catalytic Reduction of NOx)

Laboratories & Research Centers
Ammonia is used in calibration gas mixtures for petrochemical industry, environmental emission monitoring, industrial hygiene monitors and trace impurity analyzers.

Metal fabrication
Ammonia is used for heat treatment: at high temperature it splits into hydrogen and nitrogen creating the necessary reducing atmosphere.
Safety & Compatibility
GHS02
Flammable
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 630 °C |
| Lower flammability limit (Chemsafe) | 14 vol% |
| Upper flammability limit (Chemsafe) | 32.5 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 651 °C |
| Lower flammability limit (NFPA 325) | 15 vol% |
| Upper flammability limit (NFPA 325) | 28 vol% |
Threshold of toxicity
| ILV-15min EU (at Patm and 293.15 K) | 36 mg/m3 or 50 ppm |
| ILV-8h EU (at Patm and 293.15 K) | 14 mg/m3 or 20 ppm |
| PEL USA OSHA (vol) | 50 ppm |
| VLEP 8h France (at Patm and 293.15 K) | 7 mg/m3 or 10 ppm |
| VLEP CT France (at Patm and 293.15 K) | 14 mg/m3 or 20 ppm |
Odor
Pungent and irritating
Metals
| Aluminium | Satisfactory |
| Brass |
Risk of stress corrosion cracking
Not recommended
|
| Monel | Satisfactory |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride |
Notable acceleration of the process of ageing and significant loss of mass
Not recommended
|
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber |
Significant loss of mass
Not recommended
|
| Chloroprene | Satisfactory |
| Chlorofluorocarbons | No data |
| Silicone |
Significant loss of mass
Not recommended
|
| Perfluoroelastomers |
Significant loss of mass
Not recommended
|
| Fluoroelastomers |
Significant loss of mass
Not recommended
|
| Neoprene | No data |
| Polyurethane |
Significant loss of mass
Not recommended
|
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass
Not recommended
|
| Fluorocarbon based lubricant | Satisfactory |
Materials compatibility
Recommendations : Air Liquide has gathered data on the compatibility of gases with materials to assist you in evaluating which materials to use for a gas system. Although the information has been compiled from what Air Liquide believes are reliable sources (International Standards: Compatibility of cylinder and valve materials with gas content; Part 1- Metallic materials: ISO11114-1 (March 2012), Part 2 - Non-metallic materials: ISO11114-2 (April 2013), it must be used with extreme caution and engineering judgement. No raw data such as these can cover all conditions of concentration, temperature, humidity, impurities and aeration. It is therefore recommended that this table is only used to identify possible materials for applications at high pressure and ambient temperature. Extensive investigation and testing under the specific conditions of use need to be carried out to validate a material selection for a given application. Contact the regional Air Liquide team for expertise service.
Learn More
More information
Gaseous ammonia was first isolated by Joseph Priestley in 1774 and was termed by him "alkaline air". Eleven years later, in 1785, Claude Louis Berthollet ascertained its composition.