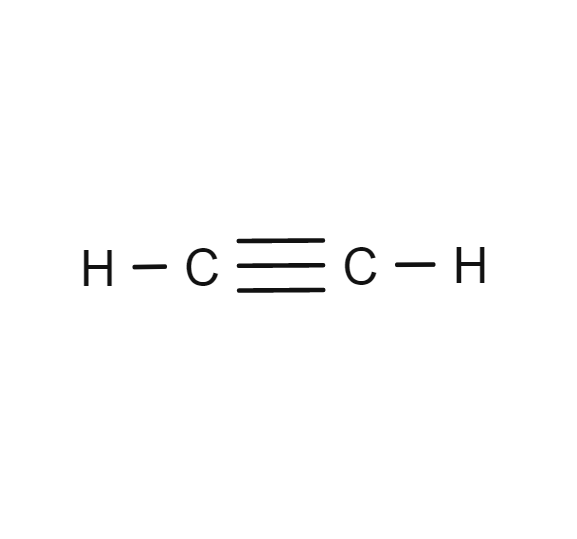
Acetylene
- C2H2
- CAS Number 74-86-2
- UN1001 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 26.037 g/mol
- Content in dry air /
-
Critical Point
- Temperature 35.15 °C
- Pressure 61.38 bar
- Density 232.48 kg/m³
-
Triple Point
- Temperature -80.75 °C
- Pressure 1.2745 bar
At triple point
| Latent heat of fusion | 144.79 kJ/kg |
| Temperature of the triple point | - 80.75 °C |
| Liquid density (at triple point) | 616.866 kg/m3 |
| Thermal conductivity | 19.298 mW/(m.K) |
| Vapor pressure | 26.4968 bar |
| Viscosity | 9.3603E-5 Po |
| Gas density | 1.171 kg/m3 |
| Temperature of the sublimation point | - 84.05 °C |
| Thermal conductivity | 20.976 mW/(m.K) |
| Vapor pressure | 38.5665 bar |
| Viscosity | 9.8768E-5 Po |
| Gas density | 1.1086 kg/m3 |
| Heat capacity Cp | 1.6913 kJ/(kg.K) |
| Thermal conductivity | 22.094 mW/(m.K) |
| Vapor pressure | 48.7024 bar |
| Viscosity | 1.0217E-4 Po |
| Gas density | 1.0707 kg/m3 |
Applications
Examples of uses of this molecule in Industry and Healthcare
Electronic components
Acetylene is used as a precursor for amorphous carbon hard mask deposition. It is also used as a carbon source for SiC and SiCN films.

Glass
Acetylene is used to lubricate the molds used in glass bottle manufacturing processes.

Hospital care
Acetylene is a component of gaseous mixtures used for pulmonary function diagnosis test.

Laboratories & Research Centers
Acetylene is used for analysis in research laboratories and quality control for industry and healthcare. Acetylene is mainly used as fuel gas for the flame required in atomic absorption spectrophotometry.

Professionnals & Craftsmen
Acetylene is used for localized or manual operations such as welding, brazing, cutting, straightening or heating.
Safety & Compatibility
GHS02
Flammable
GHS04
Gas under pressure
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Autoignition temperature (Chemsafe) | 305 °C |
| Lower flammability limit (IEC 80079-20-1) | 2.3 vol% |
| Upper flammability limit (IEC 80079-20-1) | 100 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Autoignition temperature (NFPA 325) | 305 °C |
| Lower flammability limit (NFPA 325) | 2.5 vol% |
| Upper flammability limit (NFPA 325) | 100 vol% |
Odor
Garlic like
Metals
| Aluminium | Satisfactory |
| Brass |
Use <65% Cu and copper alloy
Acceptable
|
| Monel | Satisfactory |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene | Satisfactory |
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride | Satisfactory |
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide |
Significant loss of mass and be careful with impurities
Acceptable
|
| Polypropylene | Satisfactory |
Elastomers
| Butyl (isobutene- isoprene) rubber | Satisfactory |
| Nitrile rubber |
significant loss of mass and be careful with impurities
Not recommended
|
| Chloroprene |
Significant loss of mass and be careful with impurities
Not recommended
|
| Chlorofluorocarbons | No data |
| Silicone |
significant loss of mass and be careful with impurities
Not recommended
|
| Perfluoroelastomers |
Significant loss of mass and be careful with impurities
Not recommended
|
| Fluoroelastomers |
significant loss of mass and be careful with impurities
Not recommended
|
| Neoprene | No data |
| Polyurethane |
Significant loss of mass and be careful with impurities
Not recommended
|
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass and be careful with impurities
Not recommended
|
| Fluorocarbon based lubricant |
Significant loss of mass and be careful with impurities
Not recommended
|
Materials compatibility
Learn More
More information
Acetylene was discovered in 1836 by Sir Edmund Davy. Acetylene is a synthesis gas generally produced from the reaction of calcium carbide with water. It used to be burnt in "acetylene lamps" to light homes and mining tunnels in the 19th century. It is colorless, unstable, highly combustible and has a strong garlic odor. It produces a very hot flame (over 3000 °C or 5400 °F) when combined with oxygen. It has been widely used for oxy-cutting and welding metal materials until it was remplaced by arc-based welding processes using argon.