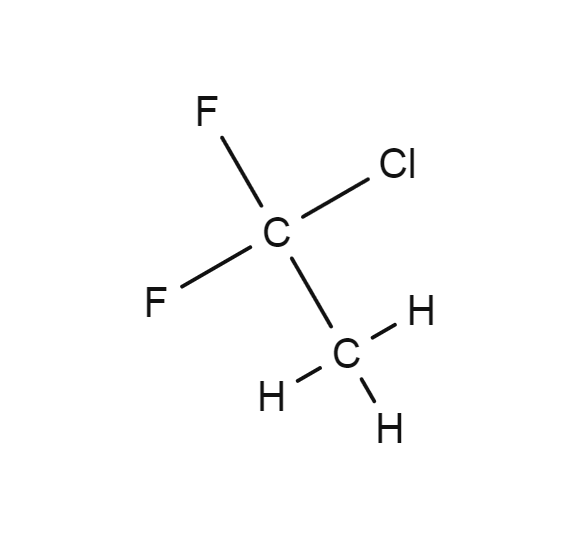
1-Chloro-1,1-difluoroethane
- C2H3ClF2
- CAS Number 75-68-3
- UN2517 (gas)
Click & drag to move the 3D molecule
Liquid / Gas Volumes
Calculate the volume or mass of a quantity of gas or liquid
Liquid Phase
At boiling point at 1.013 bar
Gas Phase
In standard conditions (1.013 bar, 15°C)
Physical Properties
Molecule phase diagram showing the transition phases between solid, liquid and gas as a function of temperature and pressure
-
- Molar mass 100.495 g/mol
- Content in dry air /
-
Critical Point
- Temperature 137.14 °C
- Pressure bar
- Density
-
Triple Point
- Temperature -130.43 °C
- Pressure 3.482E-5 bar
Pressure 1.013 bar
| Latent heat of fusion (at melting point) | 26.728 kJ/kg |
| Melting point | - 130.8 °C |
Pressure 1.013 bar
| Boiling point | - 9.12 °C |
Applications
Examples of uses of this molecule in Industry and Healthcare

Chemicals
1-Chloro-1,1-difluoroethane (R142B) is a blowing agent component for polyurethane and for extruded polystyrene foams.
Safety & Compatibility
GHS04
Gas under pressure
Autoignition Temperature, Flammability Limits & Flash Point
Europe (according to EN1839 for Limits and EN 14522 for autoignition temperature)
| Lower flammability limit (IEC 80079-20-1) | 6.3 vol% |
| Upper flammability limit (IEC 80079-20-1) | 17.9 vol% |
US (according to ASTM E681 for Limits and ASTM E659 for autoignition temperature)
| Lower flammability limit (NFPA 325) | 6.2 vol% |
| Upper flammability limit (NFPA 325) | 17.9 vol% |
Odor
Slightly ethereal
Metals
| Aluminium | Satisfactory |
| Brass | Satisfactory |
| Monel | No data |
| Copper | No data |
| Ferritic Steel | Satisfactory |
| Stainless steel | Satisfactory |
| Zinc | No data |
| Titanium | No data |
Plastics
| Polytetrafluoroethylene | Satisfactory |
| Polychlorotrifluoroethylene |
Significant swelling
Acceptable
|
| Polyvinylidene fluoride | Satisfactory |
| Polyvinyl chloride |
Significant swelling
Not recommended
|
| Ethylene tetrafluoroethylene | No data |
| Polycarbonate | No data |
| Polyamide | Satisfactory |
| Polypropylene |
Significant swelling
Not recommended
|
Elastomers
| Butyl (isobutene- isoprene) rubber |
Significant swelling
Acceptable
|
| Nitrile rubber |
Significant swelling
Acceptable
|
| Chloroprene |
Significant swelling
Acceptable
|
| Chlorofluorocarbons | No data |
| Silicone |
Significant swelling
Not recommended
|
| Perfluoroelastomers |
Significant swelling
Not recommended
|
| Fluoroelastomers |
Significant swelling
Not recommended
|
| Neoprene | No data |
| Polyurethane |
Significant swelling
Not recommended
|
| Ethylene-Propylene | Satisfactory |
Lubricants
| Hydrocarbon based lubricant |
Significant loss of mass
Not recommended
|
| Fluorocarbon based lubricant |
Significant loss of mass
Not recommended
|
Materials compatibility
Learn More
More information
Due to their ozone-depleting effect, the production of refrigerants is continuously decreasing, based on Montreal protocol requirements. Their use is controlled and they are progressively being replaced.